Attention was directed towards our fossil fuel dependency and the vulnerability of our energy system in 1973 with the oil crisis. This again occurred after the turn of the millennium with increased focus on climatic changes due to increasing levels of carbon dioxide in the atmosphere. Since the start of industrialization, the global energy demand has increased exponentially, partly due to the expanding world population. However, we have plenty of available renewable energy resources, such as sunlight and wind, but these fluctuate strongly over time and geography. The most difficult challenge appears to be efficient, reliable and long-term storage of renewable energy, over days, weeks and maybe months, which can level out the fluctuating energy sources and integrate with our varying energy consumption.
Today, a sustainable energy system based on renewable energy has a high position on the long list of societal challenges we are facing and scientists have significant responsibility to contribute. We need fundamentally new and ground breaking ideas, not just incremental improvements of known technologies in order to create a sustainable energy system based on renewable energy. Hydrogen and metal hydrides may be of key importance in designing new energy storage and conversion technologies, also providing the potential of closing the carbon cycle.
The aim of this combined symposium and summer school is to combine the knowledge from experienced scientists with the creativeness and scientific curiosity from young scientist. Many high profile invited speakers will present talks starting with an introductory component, followed by frontier research results and ending with a discussion of possible future utilization.
In the first talks, the present fossil fuel based energy system will be discussed along with new possibilities to close the carbon cycle. Recently, it was demonstrated that metal hydrides react with carbon dioxide and form e.g. methanol or methane. Hydrogen is readily produced by the electrolysis of water, and can be used directly as a fuel for cars. Hydrogen may be stored in a very dense way in the solid state, which has been researched during the past decades. The most prominent results using Mg, Al, B, N as hydrogen carriers will be discussed, along with reversible reactive hydride composites.
The question is: Can we include carbon or other lighter elements directly in new types of hydrogen storage systems? Lectures on new hydrogen storage systems will shed more light on this issue.
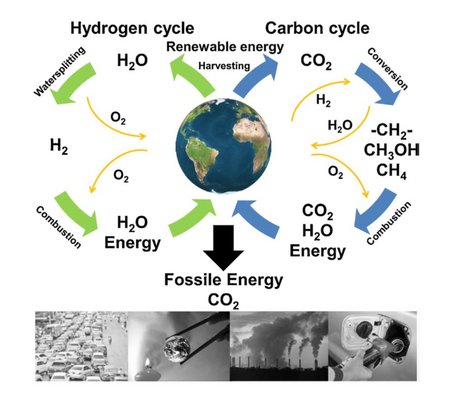
The Figure [1] illustrates that water can be ‘split’ into oxygen and hydrogen (green arrows) and most of the energy used in this process can be released again when hydrogen reacts with oxygen.
A carbon-based energy carrier system is also illustrated (blue arrows) but hydrocarbons are currently energy-consuming and difficult to produce from biomass or CO2 from the atmosphere.
Notice, a sustainable future can only be created with closed materials cycles for all chemicals and materials that we use. Today, most materials are unfortunately ‘single-use’ and not re-cycled. Likewise, CO2 produced from fossil fuels is discarded into the atmosphere symbolized by personal vehicles and industrial emission ultimately leading to global warming (black arrow, picture row)
[1] "Complex hydrides for hydrogen storage – new perspectives", Morten B. Ley, Lars H. Jepsen, Young-Su Lee, Young Whan Cho, José Bellosta von Colbe, Martin Dornheim, Masoud Rokni, Jens Oluf Jensen, Michael Sloth, Yaroslav Filinchuk, Jens Erik Jørgensen, Flemming Besenbacher, Torben R. Jensen*, Mater. Today, 2014, 17(3), 122-128
http://www.sciencedirect.com/science/article/pii/S136970211400073X (Open Access)